
More than 30 percent of the world’s land area is made up of forests, and there are estimated to be 3 trillion plus trees on Earth. In their natural state, trees and other woody plants are vital for all aspects of life on earth. This includes the absorption of carbon dioxide, providing habitats for countless species of animals and other organisms, preventing soil erosion, and providing filtration of vital air and water sources.

However, biomass from trees can be used in a number of ways that you may not have expected, especially to support green and sustainable chemistry. Read on to find out more.
The demand for battery materials is growing every day, and one solution for this is a renewable source of carbon to produce graphite—a vital component of many lithium-ion batteries.
Graphite is made up of layered graphene—polymers of pure carbon, arranged in hexagonal sheets. This arrangement allows electrons to flow readily from one source to another, making them the perfect electrodes inside the batteries needed for electric vehicles and other battery-powered devices. Graphite is very tough and stable, and can be useful for long periods of time without degrading. The substance occurs naturally, but the demand for material volume and purity has seen different synthetic routes become more mainstream. These methods often source carbon from fossil fuels such as methane, and require an abundance of energy, thus minimising the functional benefits of using batteries at all.
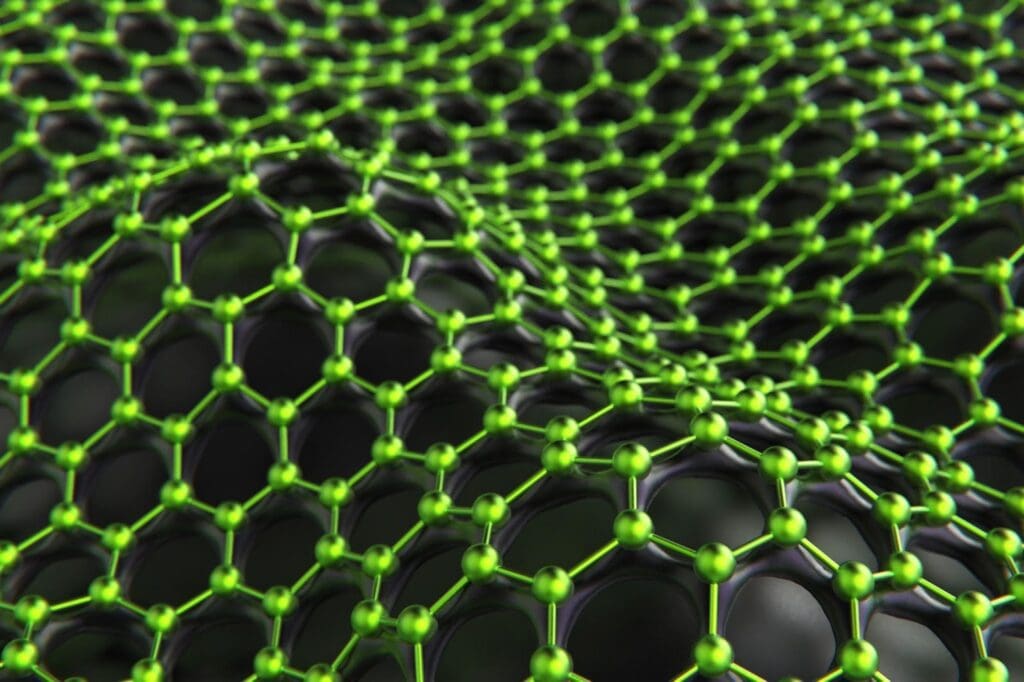
Woody biomass is primarily made up of lignin and cellulose—the two most abundant polymers on earth—which are primarily composed of carbon, with some hydrogen and oxygen. These complex organic polymers can be broken down or rearranged into many other useful molecules, including graphite and graphene, in lieu of unsustainable fossil fuels.
With the right combination of treatment and catalysts, lignin and cellulose can be used to synthesise many commercially valuable chemicals, which previously have been derived from fossil fuels. A new study published in the Journal of the American Chemical Society found that solid acid catalysts, such as zeolites and inorganic salts, can effectively synthesise acrylic acid from lactic acid, with a conversion rate as high as 92%.
Lactic acid is a common by-product from the breaking-down of lignocellulosic biomass from trees and other woody plants. Acrylic acid and other acrylates are key industrial chemicals, commonly used in adhesives, paints and polishes, superabsorbent materials, and as a feedstock for other key polymers and plastics. Not only is this new catalytic route more sustainable than fossil fuel-derived acrylic acid, it is also potentially more cost effective—which is one of the biggest drawbacks of developing new sustainable processes.
Fuel is perhaps the most promising use of biomass, as a renewable replacement for petroleum. In theory, biofuels can be burned for energy and remain carbon neutral (or even carbon negative) due to the carbon absorption as part of plant growth.

There are three different categories of biofuel, depending on the origin of the plant matter. First generation biofuels are sourced from existing food crops, such as corn or soy, and require relatively little processing to turn into a viable fuel source such as ethanol or oils. One drawback, however, is the limited amount of arable land available on Earth. To provide enough crops for both food and fuel purposes, the layout of global farmland needs to be optimised, as well as the use of resources such as water and fertilisers.
Woody biomass is considered a second-generation biofuel, as it often comes from the waste of existing processes such as paper manufacture or timber processing. Because this is mostly made of lignin and cellulose, more energy is required to break these down into simpler hydrocarbons which can then be used for fuel. Second generation biofuels can also be made from agricultural waste, like wheat straw or corn stalks, once they have served their purpose as food crops.
The third generation of biofuels comes from oil-producing algae, which require dedicated facilities to produce fuel feedstocks. Once the oil is acquired from the algae, manufacture into fuel is relatively easy. However, achieving optimal algae growth is challenging and expensive.
Concerned about your chemical processes? We are here to help. At Chemwatch we have a range of experts spanning all chemical management fields, from chemical storage to Risk Assessment to heat mapping, eLearning and more. Contact us today to find out more!
Sources: