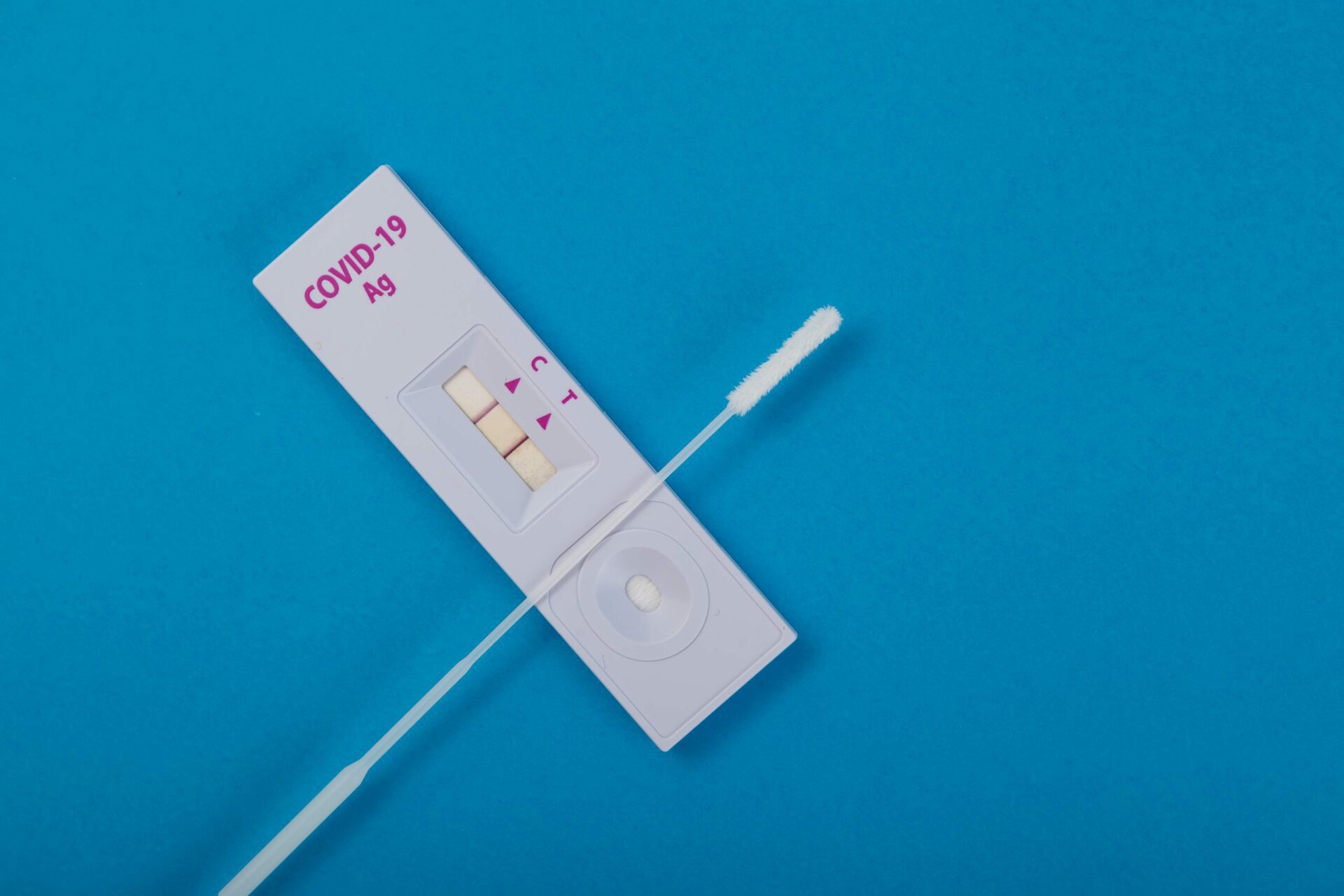
Lateral flow immunochromatographic assays, more commonly known in Australia as Rapid Antigen Tests (RATs), are a hot commodity. Alongside vaccines, they have been hailed as a saving grace in the fight against COVID-19, assisting nations as they attempt to move beyond lockdowns and enter the endemic phase – living with the virus. With everyone scrambling to get their hands on the elusive little kits, we thought it high time to explain how these tests are able to yield their all-important results.
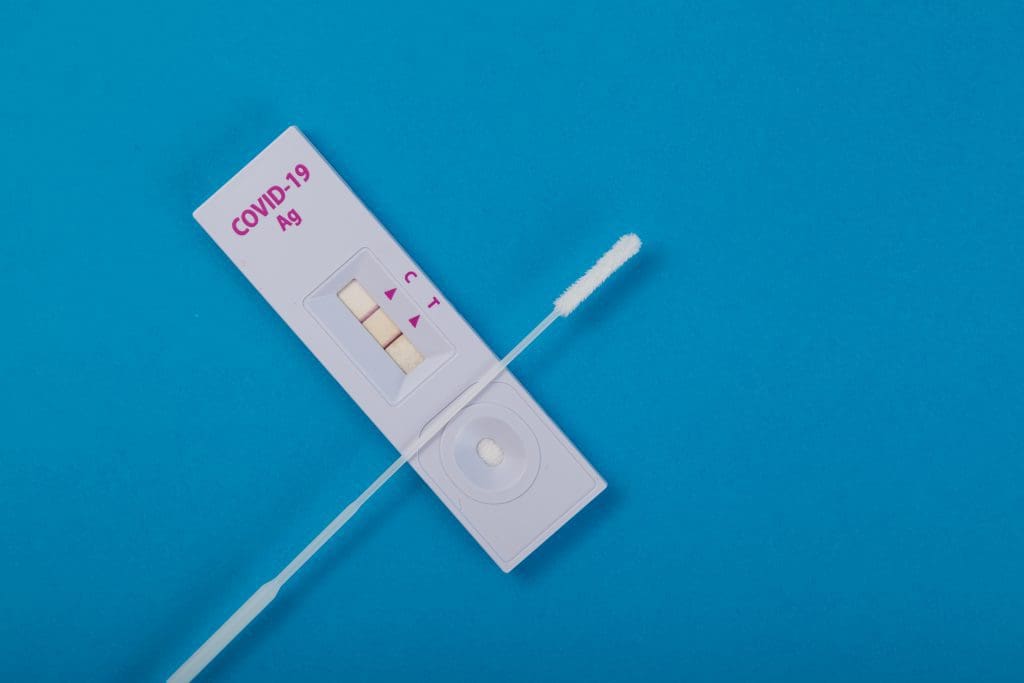
RATs are able to give their timely results using an analytical method known as an assay. Prior to the COVID-19 pandemic, the most commonly seen application of this method in the home was a pregnancy test, using the assay to detect certain hormones in a urine sample. The method runs a liquid sample along an absorbent bed to the testing site where a chemical reaction takes place, indicating the presence or absence of a target molecule. In the case of COVID-19, this will detect antigens – molecules that evoke an immune response in the body. Other kinds of assay tests might pick up on antibodies (targeted molecules created by the immune system to defend against antigens), nucleic acids, or hormones.
The assay uses a control line and a test line to confirm that the test has worked properly, that the sample has been absorbed and the detectors are functioning. A valid test will always show a control line. The test line is the key to the result, where receptors are present on the absorbent pad to bind to antigens within the sample. If there are no antigens detected (or not enough), no line will appear.
PCR, or polymerase chain reaction, is a DNA-specific test, rather than an antigen test. Antigens can take days to appear in the bloodstream of an infected person, whereas viral DNA within host cells can be detected much sooner. The PCR method takes a DNA sample and replicates it millions or even billions of times to easily find anomalies, such as viral DNA or RNA, within a tiny sample size.
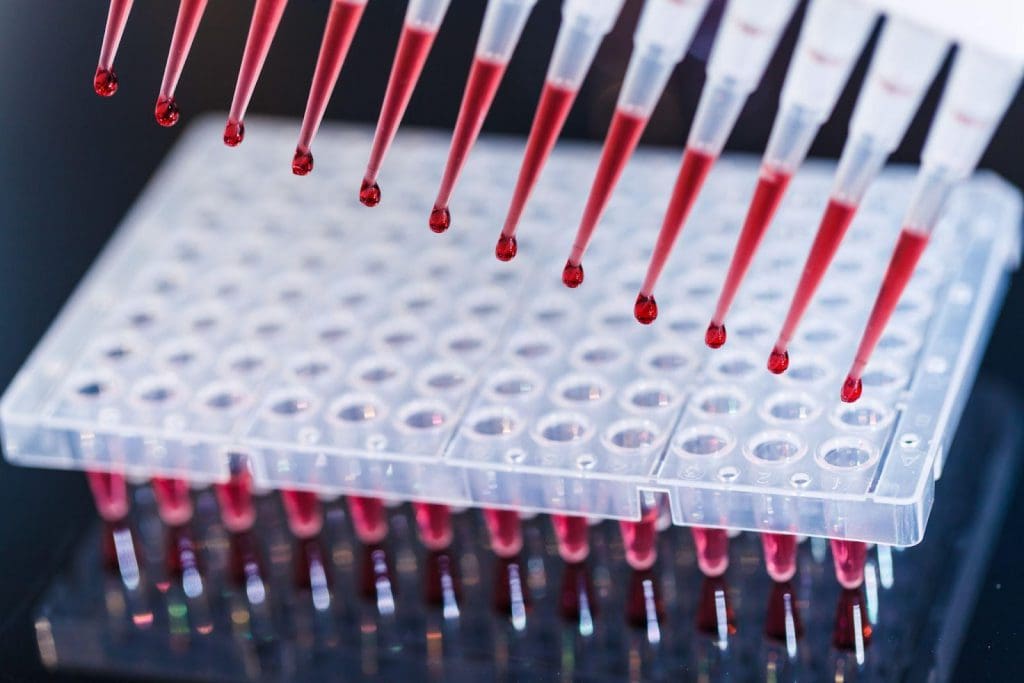
PCR tests are used in all sorts of diagnostic applications, from infectious diseases to cancer to genetic changes and anomalies. In addition to the very small sample size needed, PCR tests also benefit from the ability to be batch-tested, which allows more tests to be done over a smaller period of time. If one batch contains traces of the target, then each sample can be individually tested, and the positive sample can be easily isolated.
Because these tests work via two different mechanisms, they have differing sensitivity (true positive rate), specificity (true negative rate), and limits of detection (the smallest amount of virus possible to detect).
The timing of the test – specifically, the window of viral load – is also very important to the tests’ accuracy, as this directly affects the limit of detection. After you become infected with COVID-19, there is an incubation period of one to three days, where the virus takes hold of host cells and begins to replicate. It can take up to five days to experience symptoms, but you can be infectious from as early as the incubation period. Because the PCR test and RAT use different mechanisms, the window for detecting COVID using a RAT is much smaller. An antigen test will typically detect COVID-19 between three to seven days after exposure, when the viral load is at a maximum. A PCR can detect COVID-19 RNA in a sample from two days up to 13. This will vary depending on your immune system, your vaccine status, and how recently you got your last vaccine or booster shot.
RATs also have a significantly lower sensitivity than PCR tests – 70-80% compared to 97% – which means the ability to detect true positives from false negatives is weaker. The selectivity of both tests is extremely high, which means that the true negative rate is very accurate, and false positives are at a minimum.
If you have any questions about COVID-19, other pathogens, or would like advice on safely handling chemical hazards, please contact the Chemwatch team today or email sa***@*******ch.net. Our friendly and experienced staff draws on years of experience to offer the latest industry advice on how to stay safe and comply with Health and Safety regulations.
Sources: