
September 2021
Commission Regulation (EU) 2020-217, the 14th adaptation to technical progress (ATP) of the CLP introduced a new harmonised classification for certain forms of TiO2 as a category 2 carcinogen by inhalation. The corresponding entry is shown in the table below:
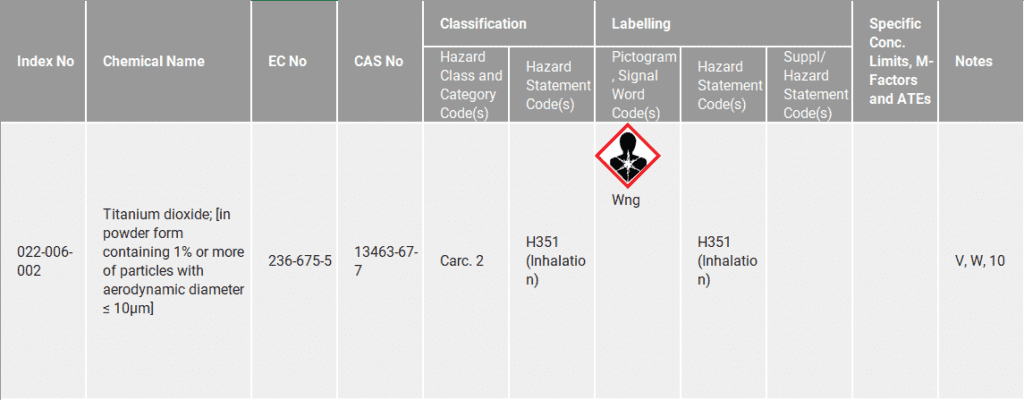
The ATP was published in the Official Journal of the EU on 18 February 2020; it came into force on 9 March 2020 and applies from 1 October 2021.
However, this classification is only applicable if:
Either the content of titanium dioxide particles with an aerodynamic diameter ≤ 10 μm is equal to or greater than 1% (w/w); or the content of titanium dioxide, which is incorporated in particles with an aerodynamic diameter ≤ 10 μm, is at least 1% (w/w).
Please keep in mind that in all cases, the total amount of titanium dioxide in the mixture, distributed among the relevant particles, must be at least 1 % (w/w).
Steps in the assessment:
| Step (1) | Check is the mixture contains 1% or more TiO2 |
| Step (2) | If yes, determine the fraction of the powdered mixture that consists of particles ≤ 10 μm |
| Step (3) | Determine the concentration (%) of TiO2 in the particles ≤ 10 μm |
| Step (4) | Calculate whether the content of TiO2 in the particles ≤ 10 μm constitutes ≥ 1 % (w/w) of the total powdered mixture. |
You have formulated a powder form mixture containing 40% (w/w) of TiO2 in the mixture. You have determined that 4% of the particles in the mixture (w/w) are within the size ranges ≤ 10 μm
Now to determine the TiO2 content in the ≤ 10 μm particles, we perform the following calculation:
(4 X 40)/100 = 1.6 % (w/w)
Therefore, the concentration of TiO2 in the mixture that is incorporated in the particles ≤ 10 μm is 1.4% and the mixture will need to be classified as Carc 2.
Consider another mixture, with 12% (w/w) of TiO2. You have determined that 8% of the particles in the (w/w) mixture are within the size range of ≤ 10 μm.
Now to determine the TiO2 content in the ≤ 10 μm particles, we perform the following calculation:
(12*8)/100 = 0.96 % (w/w)
Therefore, the concentration of TiO2 in the mixture that is incorporated in the particles ≤ 10 μm is 0.96% and the mixture will not need to be classified as Carc 2.
Part 2 of Annex II to the CLP Regulation is mandatory, in accordance with Article 25 (6).- Applied to solid and liquid mixtures containing TiO2, if they contain titanium dioxide that is hazardous or may become hazardous during the formulation of a mixture.
A solid mixture may occur in various forms, such as a mixture in powder form or as polymer pellets incorporating titanium dioxide, or as pressed blocks.
EUH212: ‘Warning! Hazardous respirable dust may be formed when used. Do not breathe dust.’
Neither the powder form nor the particle size shall be taken into account for the application of EUH212.
Liquid mixtures containing titanium dioxide do not require classification as Carc. 2, H351(inhalation).
EUH211: ‘Warning! Hazardous respirable droplets may be formed when sprayed. Do not breathe spray or mist.’
Whether a liquid mixture meets the requirements for supplemental labelling should usually be calculated based on the ingredient substances used to formulate the mixture. To do so, it seems pragmatic to assume that the particles do not change when the ingredient substances are mixed to form a liquid. However, suppliers of ‘ingredient substances’ can document if such changes are likely to occur, for example, when formulating paint dispersions.
The label on the packaging of liquid and solid mixtures that are not intended for the general public and have not been classified as hazardous and labelled with EUH211 or EUH212 shall also bear the EUH210 ‘Safety Data Sheet available on request.